There is a statewide need for the production of personal protective equipment (PPE). Our goal is to be a clearinghouse for information about safe production process and what you can deliver that will be accepted by all of the varied essential personnel who might need PPE.
Below you can find out about PPE types that you can help to produce and what level of development each is at. DFab and UW Medicine have been working together to iterate on and develop safe small-scale production options that are FDA approved, either cast or injection molded options for large scale production, and process and labeling guidelines. Each PPE type is at a different stage on this front.
Face Shields: FDA Vetted

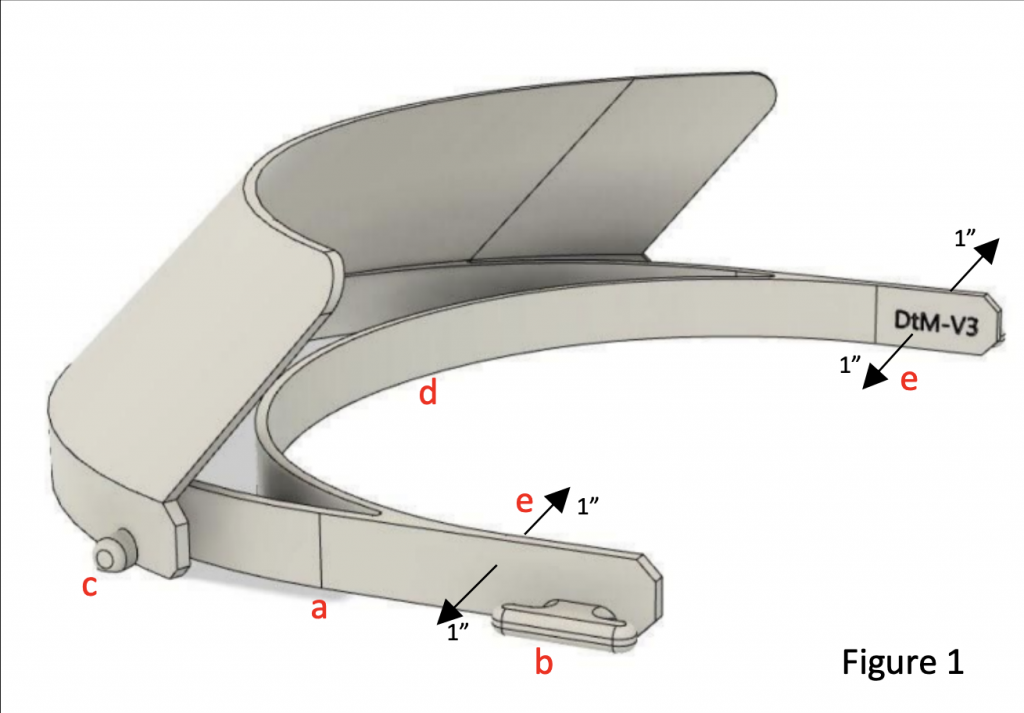
Currently, the “DtM-v3.1” design (from Prusa, evolved by designthatmatters.org) is FDA vetted and recommended for clinical use by the NIH.
Production Instructions
When produced following proper process and delivered with correct labeling and quality control documentation as described on our Face Shield Process page, this can be donated to people who need face shields. [Full Production Instructions]
More on face shields for makers. News about face shields.
Gowns: Final Design Iteration

Currently, the “UW-DFab Isolation Gown” has not yet been assessed for community use or in a clinical setting. However it has been submitted for assessment to the NIH 3D Print exchange.
